Hickey, J., et al. Two recombinant cytomegalovirus antigens formulated with the SPA14 adjuvant system: Impact of temperature, pH and excipients on the stability of each antigen and adjuvant component.
J Pharm Sci, 2025 Feb, 114(2):1224-1236. PMID: 39864550
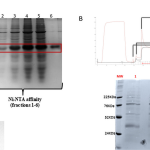
The study evaluated the stability of two recombinant Cytomegalovirus antigens (gB and Pentamer) formulated with the SPA14 adjuvant system, a liposomal formulation containing QS-21 and the synthetic TLR4 agonist E6020. Analytical characterization and accelerated stability studies were conducted under various conditions (temperature, pH, and excipients) to identify formulation parameters enhancing stability.
The SPA14 adjuvant system induced a balanced Th1/Th2 immune response when co-formulated with CMV antigens. The presence of antigens did not significantly alter liposome particle size. However, minor pH changes during storage negatively affected liposome integrity and QS-21 stability but had minimal impact on E6020. Additionally, trace metal ions contributed to the instability of QS-21 and E6020; thus, adding metal chelators and free radical scavengers improved their stability.
This work underscores the need for targeted formulation optimization to mitigate degradation pathways and improve the overall stability of vaccine candidates.
Click here to access the full scientific paper.