Haine, V., et al. Safety of RTS,S/AS01E malaria vaccine up to 1 year after the third dose in Ghana, Kenya, and Malawi (EPI-MAL-003): a phase 4 cohort event monitoring study.
Haine, V., et al. Safety of RTS,S/AS01E malaria vaccine up to 1 year after the third dose in Ghana, Kenya, and Malawi (EPI-MAL-003): a phase 4 cohort event monitoring study.
Lancet Glob Health, 2025 Apr 24, S2214-109X(25)00096-8. PMID: 40288377
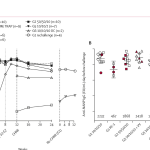
- The EPI-MAL-003 study evaluates the safety and effectiveness of the RTS,S/AS01 malaria vaccine in real-world settings across Ghana, Kenya, and Malawi, one year after the third dose was administered through the Malaria Vaccine Implementation Programme (MVIP). It included an analysis of 44,912 children and employed enhanced surveillance and monitoring systems for safety events, particularly focusing on meningitis, cerebral malaria, and mortality rates.
- The RTS,S/AS01 vaccine has been shown to provide significant efficacy in reducing malaria incidence among young children. In pivotal phase 3 trials, the vaccine demonstrated an efficacy of approximately 51% against clinical malaria during the 12 months following the third dose and a median efficacy of 36% over a longer follow-up period of 48 months, averting an average of 1774 cases of clinical malaria per 1000 vaccinated children.
- This study is the largest observational study to date on malaria vaccination outcomes founding high vaccine coverage and no evidence of increased safety risks. The results indicate a favorable safety profile for RTS,S/AS01E. The RTS,S vaccine adds a complementary approach to malaria control strategies, and therefore, in regions with moderate to high transmission of Plasmodium falciparum, the RTS,S vaccine is intended to serve as an additional tool alongside the insecticide-treated nets (ITNs) and indoor residual spraying (IRS) conventional methods. The data suggest that the RTS,S/AS01 vaccine not only remains safe but also contributes to reducing malaria-related deaths in endemic regions, supporting its continued use in young children.
Click here to access the full scientific paper.
Recent Posts