Venkatraman, N., et al. Evaluation of a novel malaria anti-sporozoite vaccine candidate, R21 in Matrix-M adjuvant, in the UK and Burkina Faso: two phase 1, first-in-human trials.
Venkatraman, N., et al. Evaluation of a novel malaria anti-sporozoite vaccine candidate, R21 in Matrix-M adjuvant, in the UK and Burkina Faso: two phase 1, first-in-human trials.
Lancet Microbe, 2025 Mar, 6(3):100868. PMID: 39805302
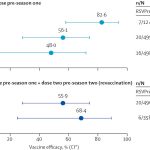
- The study evaluates the safety and immunogenicity of the novel malaria vaccine candidate R21, administered with the Matrix-M adjuvant, in two phase 1 clinical trials conducted in the UK and Burkina Faso. The trials aimed to assess the tolerability of different doses of the vaccine and the resulting immune responses in malaria-naïve individuals and healthy adults from a high malaria transmission area.
- Results indicated that R21/Matrix-M elicited strong antibody responses and had an acceptable safety profile, with minimal reactogenicity and no significant post-vaccination fever, which is a crucial benefit for mass immunization campaigns. The study highlighted that the saponin-based Matrix-M adjuvant exhibited an improved safety profile compared to the RTS,S vaccine, which contains a different adjuvant (AS01E).
Click here to access the full scientific paper.
Recent Posts