Ramezani-Rad, P., et al. The saponin monophosphoryl lipid A nanoparticle adjuvant induces dose-dependent HIV vaccine responses in nonhuman primates.
Ramezani-Rad, P., et al. The saponin monophosphoryl lipid A nanoparticle adjuvant induces dose-dependent HIV vaccine responses in nonhuman primates.
J Clin Invest, 2025 Mar 4, 135(8):e185292. PMID: 40036068
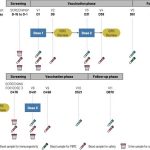
- The study investigates the induction of HIV broadly neutralizing antibodies (bnAbs) through an innovative vaccine approach using targeted adjuvants, specifically QS-21 saponin-based nanoparticle vaccines (SMNP). Conducted in nonhuman primates, it assesses the immune responses elicited by varying doses of QS-21 SMNP and evaluates the formation of germinal centers, B cell memory, and T cell activation associated with effective humoral immunity against HIV. The findings highlight the importance of dosing strategies and adjuvant formulations in optimizing vaccine-induced B cell responses and suggest that targeted approaches could enhance the development of an effective HIV vaccine.
- Immunogenicity: The QS-21 SMNP nanoparticle vaccines elicited robust immunogenic responses in nonhuman primates, characterized by the induction of high frequencies of antigen-specific CD4+ T cells and durable antibody responses. Notably, the QS-21 SMNP formulation enhanced the creation of B cell memory and plasma cells specific to HIV, with higher doses resulting in stronger and more sustained immune responses. The vaccine effectively induced not only humoral immunity, evidenced by neutralizing antibody production, but also cellular immunity, with significant T cell activation detected across various doses.
- Safety profile: QS-21 SMNP was found to be well-tolerated in nonhuman primates throughout the study. The vaccine did not exhibit significant adverse effects, suggesting that it is a safe option for inducing immune responses. Furthermore, the reactogenicity associated with QS-21 SMNP was attributed to the effective induction of immune responses without overwhelming systemic reactions. The results indicate that even at the highest tested doses (400 μg), QS-21 SMNP demonstrated a favorable safety margin, supporting its advancement towards human clinical trials.
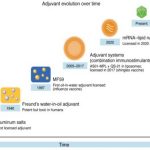
- The importance of QS-21 was highlighted as a critical vaccine adjuvant due to its potential to enhance immune responses, particularly in the context of HIV vaccines. The paper emphasizes its ability to induce strong B cell and T cell responses, which are essential for effective vaccination against difficult pathogens like HIV.
Click here to access the full scientific paper.
Recent Posts