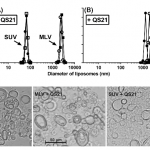
QS21 Nano Patch Queensland U 2016
Potent response of QS-21 as a vaccine adjuvant in the skin when delivered with the Nanopatch, resulted in adjuvant dose sparing.
Hwee-Ing Ng, Germain J. P. Fernando, Alexandra C. I. Depelsenaire & Mark A. F. Kendall
Adjuvants play a key role in boosting immunogenicity of vaccines, particularly for subunit protein vaccines. In this study we investigated the induction of antibody response against trivalent in uenza subunit protein antigen and a saponin adjuvant, QS-21. Clinical trials of QS-21 have demonstrated
the safety but, also a need of high dose for optimal immunity, which could possibly reduce patient acceptability. Here, we proposed the use of a skin delivery technology – the Nanopatch – to reduce both adjuvant and antigen dose but also retain its immune stimulating e ects when compared to the conventional needle and syringe intramuscular (IM) delivery. We have demonstrated that Nanopatch delivery to skin requires only 1/100th of the IM antigen dose to induce equivalent humoral response. QS-21 enhanced humoral response in both skin and muscle route. Additionally, Nanopatch has demonstrated 30-fold adjuvant QS-21 dose sparing while retaining immune stimulating e ects compared to IM. QS-21 induced localised, controlled cell death in the skin, suggesting that the danger signals released from dead cells contributed to the enhanced immunogenicity. Taken together, these ndings demonstrated the suitability of reduced dose of QS-21 and the antigen using the Nanopatch to enhance humoral responses, and the potential to increase patient acceptability of QS-21 adjuvant.