Leroux-Roels, I., et al. Safety and Immunogenicity of an Adjuvanted Clostridioides difficile Vaccine Candidate in Healthy Adults: A Randomized Placebo-Controlled Phase 1 Study.
Leroux-Roels, I., et al. Safety and Immunogenicity of an Adjuvanted Clostridioides difficile Vaccine Candidate in Healthy Adults: A Randomized Placebo-Controlled Phase 1 Study.
J Infect Dis, 2025 Mar 17, 231(3):e511-e520. PMID: 39447053
- The study investigated the safety and immunogenicity of GSK’s Clostridioides difficile vaccine candidate, known as GSK2904545A, in healthy adults aged 18-70. Participants received F2 antigen with or without the AS01 adjuvant in a randomized, placebo-controlled, phase 1 trial.
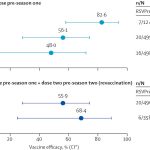
- Immunogenicity: Subjects receiving the F2 antigen combined with the AS01 adjuvant exhibited significantly higher neutralization titers against the Clostridioides difficile toxins TcdA and TcdB compared to those receiving the F2 antigen alone. Neutralization titers peaked after the second dose but declined before the third dose, after which a substantial increase in titers was observed, particularly in subjects with baseline neutralization titers below the lower limit of quantitation (LLOQ). The third dose notably enhanced the immune response in these participants, highlighting the potential of the vaccine candidate to elicit a robust immune response against C. difficile infection.
- Safety profile: Overall, the vaccine demonstrated a favorable safety profile with no significant safety concerns raised during the study. Most reported adverse events were mild to moderate in severity and were transient, with the most common AEs being injection site pain, fatigue, and headaches. No serious adverse events were deemed related to the vaccine.
- The adjuvant AS01, particularly its saponin component, is crucial for maximizing the effectiveness of the vaccine candidate by enhancing the immune response while also contributing to the overall reactogenicity. Overall, the study suggests that the F2 antigen vaccine candidate is promising for preventing C. difficile infections, particularly when combined with AS01.
Click here to access the full scientific paper.
Recent Posts