Hu, J., et al. Herpes simplex virus 1 fusion glycoprotein B H516P prefusion mutation had no effect on vaccine immunogenicity.
Hu, J., et al. Herpes simplex virus 1 fusion glycoprotein B H516P prefusion mutation had no effect on vaccine immunogenicity.
Vaccine, 2025 May 31, 57:127241. PMID: 40354699
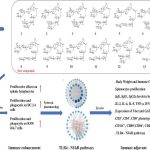
- This study investigates the effect of the H516P mutation in HSV-1 glycoprotein B (gB), designed to stabilize the protein’s prefusion conformation, on vaccine immunogenicity. The immune responses of both mRNA and adjuvanted subunit vaccines containing either the wild-type or mutated gB were evaluated in mice.
- The main effect of QS-21, as reported in the study, is its ability to significantly enhance cellular immune responses. Specifically, QS-21 increased the production of IL-2 and IFN-γ by CD4+ and CD8+ T cells, as shown by flow cytometry analyses. Compared to alum-adjuvanted vaccines, the combination of QS-21 with CpG ODNs elicited higher levels of T-cell responses, including increased proportions of IL-2-producing T cells, up to 0.15% for CD4+ T cells and 0.13% for CD8+ T cells. This indicates that QS-21 boosts T-cell activation and cytokine production, which are essential for effective cellular immunity against HSV-1.
- The mRNA vaccine elicited a cellular immune response level comparable to that induced by the subunit vaccine combined with QS-21 and CpG ODNs.
- The results demonstrated that the H516P mutation did not significantly alter humoral or cellular immunity compared to the wild-type. Additionally, the study found that combining adjuvants QS-21 and CpG ODNs augmented cellular immune responses more effectively than aluminum. Importantly, all vaccinated mice were protected from infection with a virulent HSV-1 strain, highlighting the potential of these vaccine strategies despite the mutation’s limited impact on immunogenicity.
Click here to access the full scientific paper.
Recent Posts