Cao, H., et al. A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice.
Cao, H., et al. A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice.
Vaccines (Basel), 2025 May 6, 13(5):497. PMID: 40432109
- This study evaluates the immunogenicity and protective efficacy of two novel HSV vaccines in mice: a QS21 + CpG-adjuvanted trivalent subunit vaccine and a trivalent HSV-2 mRNA vaccine, both targeting gC2, gD2, and gE2 glycoproteins.
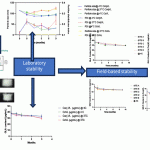
- Both the QS21 + CpG-adjuvanted subunit vaccine and the mRNA vaccine effectively elicited strong antigen-specific humoral responses, as well as robust cellular immune responses.
- QS-21, when combined with CpG oligonucleotides, synergistically stimulates innate immune pathways, leading to a robust activation of antigen-specific T cells, particularly Th1-type responses, and the production of high levels of cytokines such as IL-2 and IFN-γ. QS21 + CpG adjuvant helps overcome the limitations of traditional aluminum adjuvants by promoting a more comprehensive immune response, including strong cellular immunity and cross-protection against related viral strains like HSV-1. Overall, QS-21 enhances vaccine immunogenicity, efficacy, and breadth of protection.
- The results demonstrate that both vaccines induce robust humoral and cellular immune responses, significantly reducing viral loads and preventing clinical symptoms following HSV-2 and HSV-1 infections, with both offering cross-protection against HSV-1, suggesting their potential for development into effective broad-spectrum herpes vaccines.
Click here to access the full scientific paper.
Recent Posts